News
Common Status and Dilemma of 2,2-Dimethylolpropionic Acid
Release time:
2020-09-19
2,2-Dimethylolpropionic acid (DMPA) can be widely used in the production of water-soluble polyurethane, polyester, epoxy resin, etc. It is a green environmental protection chemical. Dimethylol propionic acid through the action of two primary hydroxyl groups, without the need to protect the third carboxyl group can be easily methyl esterification or esterification of carbamate, and then in ammonia to neutralize the unreacted carboxyl group, so that it is water soluble. In terms of solubility, dimethylolpropionic acid has its own unique advantages, and its excellent solubility can greatly improve work efficiency.
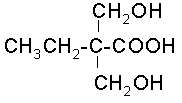
Due to the difficulty of preparation technology, some current synthesis technologies also have problems such as poor catalyst selectivity and reactivity, complex process routes, high energy consumption, and serious environmental pollution.
At present, the synthesis of 2,2-dimethylol propionic acid at home and abroad is mainly carried out in two steps. One is to use hydrogen peroxide as an oxidant, after 2,2-dimethylolbutyraldehyde oxidation, crystallization to obtain 2,2-dimethylolpropionic acid white crystal; two is in the presence of alkaline catalyst, formaldehyde and butyraldehyde aldol condensation reaction to generate 2,2-dimethylolbutyraldehyde. The aldol condensation reaction is the key step of the two-step method, and the alkaline catalyst is mostly inorganic alkali (Na2CO3, NaOH). Due to the occurrence of side reactions such as Conizaro reaction in the condensation reaction process, the pH value of the reaction system changes greatly, and there are problems such as poor catalyst selectivity, poor reactivity and low product yield.

After the oxidation of 2,2-dimethylol propionaldehyde, white crystals of 2,2-dimethylol propionic acid were obtained by organic solvent (methanol, dichloroethane, etc.). (Note: Because the organic solvents such as methanol and dichloroethane are mostly flammable and explosive substances, the safety is relatively poor, and it is toxic and harmful to the environment and human body), the crude product after crystallization is centrifuged to obtain dimethylol propionic acid.
At present, the industrial research on the preparation of 2,2-dimethylolpropionic acid is based on the two-step process, which focuses on the catalyst selection of aldol condensation reaction and the selection of reaction conditions, but all because of the existence of side reactions such as Conizaro reaction, the problems such as poor control of production process, poor degree of process optimization, low product yield, high energy consumption of products, and serious discharge of product pollutants (especially crystallization mother liquor wastewater) have not been solved.
Preparation method of 2,2-dimethylol propionic acid
Previous Page
Share











